Define Polarizability and Explain How It Is Different From Polarity
The polarizing power of a cation is proportional to its charge radius ratio. Define electronegativity and explain how it provides a measure for the polarity of a chemical bond.

Dipole Moment Molecular Polarity Percent Ionic Character Youtube
Thus molecules attract one another more strongly and melting and boiling points of covalent substances increase with larger molecular mass.

. As polarizability increases the dispersion forces also become stronger. We have to distinguish between polar provides a busy and also polarity. It is a property of all matter considering that matter is made up of elementary particles which have an electric charge namely protons and electrons.
On the other hand polarizability is an ability property of a molecule. It describes how easy for a molecule to be polarized change of overall dipole moment by external electric field. Most of these materials are solid in nature and some fluids and gases exhibit dielectric properties.
Polarity is a physical property of compounds which relates other physical properties such as melting and boiling points solubility and intermolecular interactions between molecules. We know that dielectrics are used in components such as capacitors and radios. For the most part there is a direct correlation between the polarity of a molecule and number and types of polar or non-polar covalent bonds.
What are the practical consequences of polarity and polarizability. The electronic polarizability is a microscopic polarization phenomena that occurs in all materials and is one of the main mechanisms that drives dielectric polarization. That quality or condition of a body in virtue of which it exhibits opposite or contrasted properties or powers in opposite or contrasted parts or directions.
When subject to an electric field the negatively charged electrons and. Define electronegativity and use this concept to explain bond polarity. Polarizability refers to the difficulty with which such a displacement can be achieved.
The units of α are C m2V-1. Polarizability which is represented by the Greek letter alpha α is experimentally measured as the ratioof induced dipole momentp to the electric field E that induces it. A molecule is basically said to be either a polar molecule non- polar molecule or ionic molecule.
AnswerA polar molecule has overall static dipole moment. How do the terms polarity and polarizability differ from each otherIn my opinion polarity is the degree of ionic character in covalent compounds and polarizability implies towards degree of covalent character in ionic compounds. To explain how the dielectric constant relates to the electronic polarizability of a material the polarization or P of a material should be determined.
When configured properly dielectric materials can be used to store energy too. Or a condition giving rise to a contrast of properties corresponding to a contrast of positions as for example attraction and repulsion in the opposite parts of a magnet the dissimilar phenomena. Join our Discord to connect with other students 247 any time night or dayJoin Here.
This is illustrated in where a large and soft anion comes under the influence of a small cation. Define electronegativity and use this concept to explain the transition between pure covalent polar covalent and ionic bonds. Were always here.
As nouns the difference between polarity and polarization is that polarity is the separation alignment or orientation of something into two opposed poles while polarization is the production or the condition of polarity. Polarazibility also affects dispersion forces through the molecular shape of the affected molecules. On the other hand polarizability explains how easily the electrons electrons cloud can be distorted or how easily a dipole can be induced in a molecule by outside forces.
Polarity is the difference in charge between the components of the molecule whereas the polarizability is the easy with which the negative and positive ends of the structure may be separated. Dielectric Polarization in Polar and Nonpolar Material and Dielectric Constant. I think the difference is small but notable.
Polarizability is the relative tendency of a charge distribution in other words the more changes in the electron cloud geometry the greater the polarizability. The direction in which the electric field of an electromagnetic wave points while polarizability is physics the relative tendency of a system of electric charges to become polarized in the presence of an external electric field. In contextphysicslangen terms the difference between polarization and polarizability is that polarization is physics the production of polarized light.
In short polarization the noun is the displacement of positive charges relative to negative charges in a system ie. Explain the difference between polarity and polarizability. Polarizability usually refers to the tendency of matter when subjected to an electric field to acquire an electric dipole moment in proportion to that applied field.
Large negatively charged ions such as I-and Br-. An atoms nucleus vs its electrons. To my understanding polarity refers to the unequal sharing of electrons or the existencestrength of a dipole in a molecule.
When a molecule is said to have. How that each of the instruments during inter molecule force so facil for our Lets look at two different Templar recipe Its a. The power of an ion to destroy the other ion is known as its polar-izing power and the tendency of the ion to the ion to distort is known as its polarizability.
A polar molecule is usually formed when the one end of the molecule is said to possess more number of positive charges and whereas the opposite end of the molecule has negative charges creating an electrical pole.
What Is The Difference Between Electric Susceptibility And Polarizabilty Quora
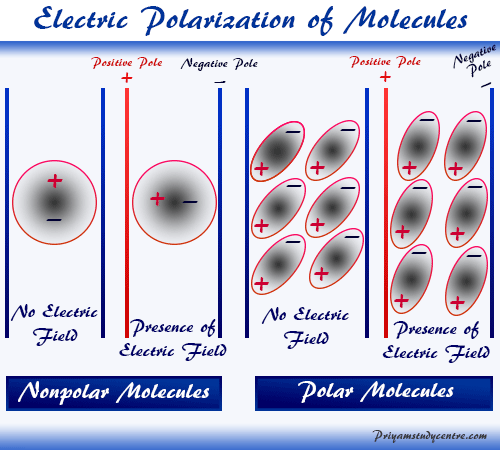
Electric Polarization Definition Types Formula Units

Polarization Definition Types Britannica

Plane Of Polarization Wikipedia

Bond Polarity Electronegativity And Dipole Moment Chemistry Practice Problems Youtube

Electric Polarization Definition Units Example Facts Britannica

Difference Between Dipole Moment And Polarisability Raman Spectra Physical Chemistry Youtube

Pin By Rishi S Classes On Van Der Waals Forces In Chemistry In 2021 Chemistry Interactive Semester

What Is Polarity Definition Example Polar Vs Non Polar Molecules

Pin By Rishi S Classes On Molecular Polarity And Weak Chemical Forces Chemistry Molecular Semester
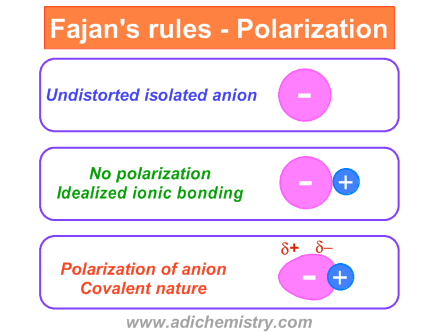
Fajan S Rules Polarization Power Polarizability Covalent Nature Of Ionic Bond
What Is The Difference Between Polarity And Polarizability Quora
What Is The Difference Between Polarity And Polarizability Quora

What Is Polarity Definition Example Polar Vs Non Polar Molecules



Comments
Post a Comment